
电化学显微镜 (EC-AFM)
带有电化学表征的表面高分辨率成像
在EC-AFM中,扫描隧道显微镜(STM)可以观察电极表面电化学反应的纳米结构。用户通常使用电化学池和根据感兴趣的电化学应用选择的恒电位仪/恒电流仪进行伏安法和腐蚀实验。

在EC-AFM中,扫描隧道显微镜(STM)可以观察电极表面电化学反应的纳米结构。用户通常使用电化学池和根据感兴趣的电化学应用选择的恒电位仪/恒电流仪进行伏安法和腐蚀实验。
电化学原子力显微镜(EC-AFM)将表面的高分辨率成像与电化学表征相结合。EC-AFM在含有电化学活性物种的液体环境中运行。一组电极允许对样品施加偏压,其中电化学反应可以根据该偏压的大小和方向发生,而AFM装置则测量表面形态及其后续变化。
这些测量是通过设计的EC电池实现的,该电池旨在同时执行EC反应,同时为成像样品表面的形貌提供合适的介质。这个电池类似于在液体环境中进行标准测量所使用的设置,主要区别在于EC电池还配备了三个电极:工作电极、对电极和参考电极(图1)。工作电极将偏置应用于(半)导电样品表面,在EC测量系统中发生感兴趣的反应。对电极用于关闭电路。其主要功能是使电流流动,例如从电解质处处置或接收电子。惰性材料(例如铂丝)通常用于对电极,以避免其表面发生不需要的副反应。参考电极作为反馈传感器,以保持施加在工作电极和对电极上的恒定电位。这个参考电极需要一个众所周知且稳定的电极电位,通常通过缓冲或浓缩的氧化还原系统(例如Ag/AgCl或H/H+)来实现。这三个电极连接到电位计,可以在监测电流流动的同时施加定义的偏置,或者在监测偏置的同时施加恒定电流,以执行典型的电化学实验,例如循环伏安法(CV)测量,用于研究电化学反应。此外,可以选择特定的偏置来强制执行样品表面的特定氧化或还原反应。工作电极和参考电极之间的高阻连接确保任何可测量的电流主要在工作电极和对电极之间流动,因此可以归因于特定的电化学反应。
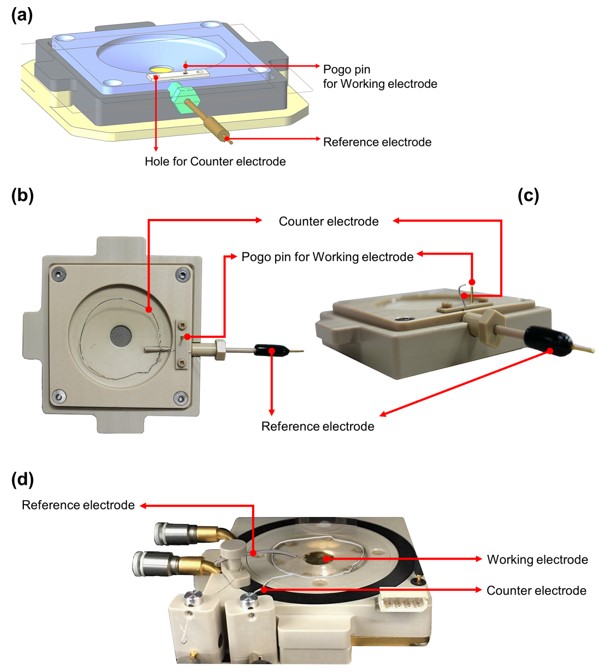
图1. EC池(a)示意图,(b)俯视图,(c)EC池侧视图,(d)带EC工具包的通用液体池。
这些电化学反应既可以通过CV测量来研究,也可以通过AFM测量来可视化样品形貌的任何变化。建议使用惰性材料制成的悬臂来避免任何副反应或探针几何形状的变化。此外,悬臂需要电绝缘,以避免不必要的杂散电流。
EC-AFM测量的一个例子是在CuSO4 /H2SO4溶液中扫描偏压时,在Au表面沉积和溶解Cu纳米颗粒。当通过工作电极施加负偏压,提供过量的电子时,Cu离子在Au表面还原形成元素Cu(方程式1)。相应地,当对Au表面施加正偏压时,元素Cu氧化,通过工作电极去除电子(方程式2)。
Cu2+ + 2e- → Cu0…… 方程式1. 还原反应,Cu沉积
Cu0 → Cu2+ - e-…… 方程式2. 氧化反应,Cu溶解
通过测量CV曲线来进行和监测这些还原/氧化反应,同时使用AFM确认Au基底上Cu颗粒的沉积/溶解。图2显示了在工作电极处于含有0.1 mM CuSO4的50 ml 0.01 mM H2SO4溶液中的四个CV曲线。在施加-0.2 V偏压时,Au表面开始形成Cu纳米颗粒,在-0.4 V时还原电流。当反向施加偏压时,这些Cu颗粒开始氧化并溶解,如从0 V开始并在约0.1 V处达到峰值的电流流动所示。在施加-0.1 V偏压时没有电流流动,表明处于平衡状态,没有发生化学反应。此外,连续四次扫描的重叠表明发生了完全可逆的氧化还原反应。
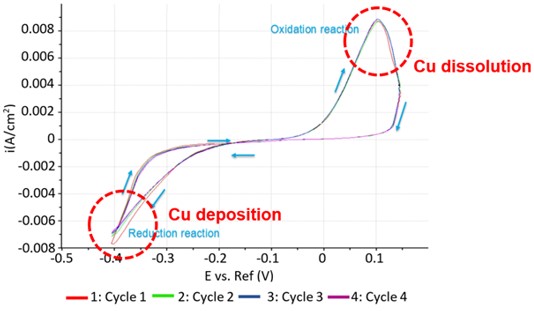
图2. Au表面可逆Cu沉积的CV曲线。
通过图3中的AFM图像确认了Cu纳米颗粒的形成和溶解,这些图像显示了Au表面在“反应前”、“Cu沉积后”和“Cu溶解后”的状态。所有图像均使用软悬臂(PPP-CONTSCR,弹簧常数为0.2 N/m,共振频率为25 kHz)在液体条件下的AFM非接触模式下获得。图3中“反应前”的AFM图像显示了一个没有任何颗粒的原始Au表面。在Cu沉积发生后,Au表面出现了Cu颗粒,如图3中“沉积”所示。这些Cu颗粒在受到正偏压后重新氧化。
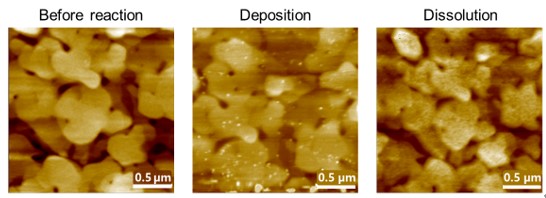
图3. Cu纳米颗粒在Au表面的沉积。原始Au表面“反应前”、在-0.4 V下还原后“Cu沉积”、
以及在0.1 V下氧化后“Cu溶解”的AFM图像。
发生的氧化还原反应的性质以及表面形态的变化可以通过参数的选择来影响。在随后的一组实验中,将-0.6 V应用于浸入Cu2+溶液中的Au表面,以沉积更大的Cu团簇(图4)。如应用3 V持续5分钟后完全暴露的Au表面所示,这种Cu团簇的形成是完全可逆的。
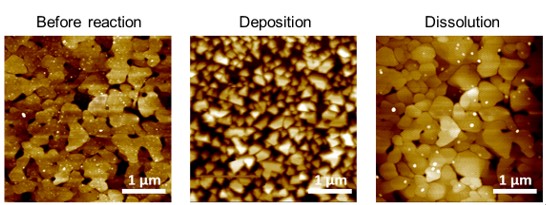
图4. Cu层在Au表面的沉积。原始Au表面“反应前”、在-0.6 V下还原后“Cu沉积”、
以及在3 V下氧化后“Cu溶解”的AFM图像。所有图像均在同一区域记录。
以下段落中介绍了EC-AFM测量的另一个例子。在这项研究中,研究了含有Li离子的电解质与Li金属在施加恒定电流密度±2 mA/cm2 时的EC反应,同时监测所需的偏压。在施加正偏压和负偏压之前和之后,使用AFM测量了Li金属表面的变化。工作电极、对电极和参比电极均为Li金属,而电解质为四乙二醇二甲醚(TEGDME)中的1M双(三氟甲磺酰基)亚胺锂(LiTFSI)。为防止Li金属在环境条件下氧化,所有测量均在充满氩气的手套箱中进行。在施加偏压之前,对原始Li金属表面进行成像,显示出光滑的表面。在施加约0.2 V的偏压后,Li表面被蚀刻,部分金属表面在5分钟内被剥离(图5)。随后,在施加负偏压的同时,在同一区域沉积了Li离子(图5)。恒电位仪能够跟踪正偏压或偏压的逐渐增加,以保持电流密度为±2 mA/cm2, 从而证实了剥离和电镀反应。
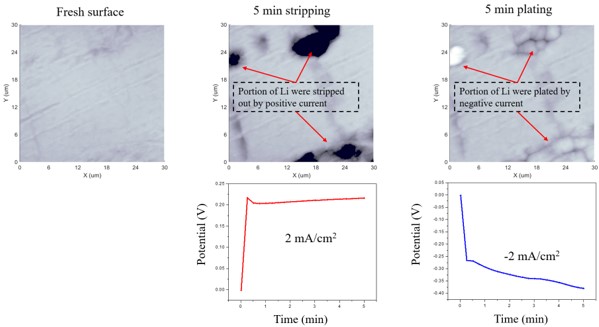
图5. Li电剥离/电镀测量
EC-AFM测量可以应用于各种研究和工业领域,因为它能够观察EC反应过程中表面形态的变化。EC-AFM在电池研究或金属腐蚀与防腐研究中尤其有用,其中EC和表面性质是关键因素。